In the near future, perfection of Organic Light Emitting Diode (OLED) technology will create an alternative to LCD screens, revolutionizing the display market. Problems associated with the emission of blue light in OLEDs currently inhibit their mass production. According to
Wikipedia.com, organic compounds that emit blue light have a lifespan of only 1,000 hours, whereas, the red and green emitting compounds have a lifespan of roughly 500,000 hours. Improvements upon the longevity of blue emitters will open a new door to display technology.
According to the article “Reliability and Degradation of Small Molecule-Based
Organic Light-Emitting Devices” in the IEEE Journal of Quantum Electronics, the cause of this limited lifespan is an undesirable reaction between oxygen molecules and the organic emitting layer. Due to the permeability of the glass substrate, oxygen molecules are able to diffuse into the organic emitting layer. During the operation of an OLED, singlet oxygen is formed due to the energy transfer from the excited organic molecules to oxygen molecules. The singlet oxygen molecules, known as radicals, then attack the structure of the organic compound eventually destroying the organic layer.
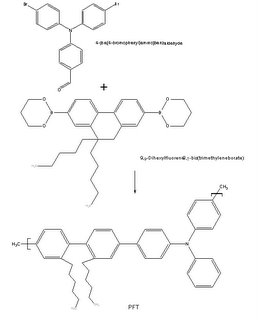
Currently, my freshman design group is working on a solution to lengthen the lifespan of the blue emitting compound. Since it is very difficult to find a blue emitting compound that does not react with oxygen at all, we decided to find a compound that reacts with oxygen, but extremely slowly. Our group is currently investigating a class of compounds known as the triarylamines, specifically, a triphenylamine-based conjugated polymer known as MPa (chosen due to MPa’s high thermal stability, low t
g, and high quantum yield of nearly 64 %). In order to create MPa we must use the Wittig Reaction. According to
Organic-chemistry.org, the Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with the ylide generated from a phosphonium salt. MPa is created by a reaction of an aldehyde group found on PFT with benzyltriphenylphosphonium bromide and THF under N
2. For a detailed description of the reaction please refer to “
Macromolecules” by the
Tokyo Institute of Technology.