Aspirin - Acetylsalicylic Acid (Thomas Dursch)
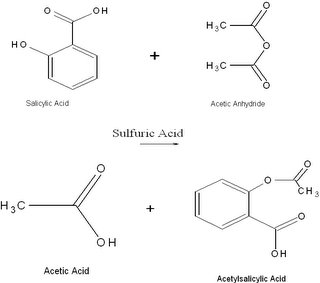
Since the time of Hippocrates, there have been written records of pain relief treatments, including powder obtained from the insides of willow trees. According to From a Miracle Drug written by Sophie Jourdier for the Royal Society of Chemistry, “It was not long before the active ingredient in willow bark was isolated; in 1828, Johann Buchner, professor of pharmaceuticals at the University of Munich, isolated a tiny amount of bitter tasting yellow crystals, which he called salicin”.
According to Wikipedia, an online encyclopedia, salicin undergoes a “Kolbe-Schmitt reaction and the final product is an aromatic hydroxyl acid known as salicylic acid”. Minimally, what happens in a Kolbe-Schmitt reaction is a sodium phenolate is heated with carbon dioxide, then the final product is treated with sulfuric acid, eventually producing salicylic acid. Today, scientists recognize salicylic acid as being harmful when ingested. Instead of using salicylic acid, the commonly used pain reliever is acetylsalicylic acid, now known as aspirin. The newly discovered acetylsalicylic acid is what is known as an “acetyl ester” formed through a process known as esterification. According to Wikipedia, for this esterification, “a carboxylic acid reacts with the phenol group of salicylic acid to produce the acetyl ester”. Simply, an ester can be thought of as a product of a condensation reaction of an acid usually an organic acid and an alcohol or phenol.
Aspirin helps cure headaches, reduce fevers, and reduce swelling to minor injuries, and since the time of its discovery, has risen to one of the leading over-the-counter drugs. According to Paul May at the University of Bristol School of Chemistry, “each year, more than 40 million pounds of aspirin is produced in the United States alone, a rate that translates to about 300 tablets per year for every man, woman and child”.
To further supplement this information and for a complete reaction/experiment, please refer to the following link.


1 Comments:
9/10 - put a link in your first paragraph for source and should have indicated where you got that pdf from
otherwise, great job!
Post a Comment
<< Home