Ozone Depletion (Alison)
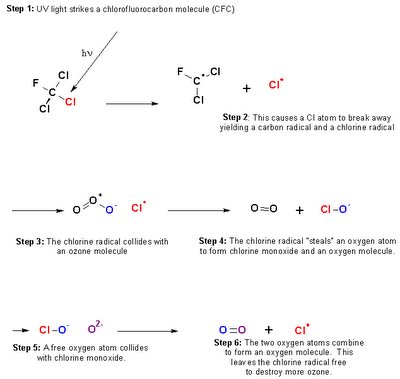
It has been known for a long time that the depletion of the ozone layer is accelerated by the use of chlorofluorocarbons, or CFC's. This reaction is cyclical, as it leaves a chlorine radical in the 6th step of halogenation to destroy more ozone molecules. This can be seen in the reaction below:
The reaction begins when UV light strikes a CFC, creating a carbon radical and a chlorine radical. The chlorine radical then collides with an ozone molecule, removes an oxygen atom, and forms chlorine monoxide and an ordinary oxygen molecule. A free oxygen atom then collides with chlorine monoxide, thus creating an oxygen molecule and freeing the chlorine radical to destroy more ozone molecules.
Beyond the obvious environmental effects of this, there are many health effects which have also been documented. The article I read focused on these health effects, mainly skin cancer, cataracts, and immune deficiencies.
Click here for CiteULike reference.
Click here for full-text of article.


1 Comments:
9/10. The only problem I see is that you did not provide a reference for steps 5 and 6. Also you have an oxygen with a double negative charge, which should be a neutral oxygen atom.
Post a Comment
<< Home